| ||||||||||||||||||||||||||||||||||
Kamis, 20 April 2017
COMPARE AND CONTRAS
Langganan:
Posting Komentar (Atom)
FENIL PROPANOID
Asal Usul Fenilpropanoid Fenilpropanoid merupakan suatu kelompok senyawa fenolik alam yg berasal dari asam amino aromatik fenilalanin ...

-
Senyawa alami secara umum adalah molekul kimia berupa mineral, metabolit primer, dan metabolit sekunder. Secara famili besar, met...
-
Classification of Matters with Examples Matter is a term used for everything having mass and volume. In this u...
-
KIMIA ORGANIK BAHAN ALAM Lipid Lipid atau lemak didefinisikan sebagai senyawa organik heterogen yang terdapat di alam dan bersifat...

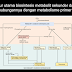

can you explain the compare and contrasct about groups in periodic table
BalasHapusargon Atomic number 18 but helium atomic number 2
Hapusargon Mass number 40 but helium massa number 4
argon Exhibiting a violet/ulac glow when placed in an elektric field but helium Exhibiting a red-orange glow when placed in an elektric field
Hiii Nadia, how are you?, I'm Novi. Want to ask you, Where can we find elements of argon and helium? It can be a differentiator.
BalasHapushay novi, i'm fine to thanks you ,We can see in peroidik table and there write no no atam and its mass
Hapuswhy you choose Ar and He for the example of your double bubble maps? why not the other elements? please explain to me
BalasHapusBecause argon and helium are both noble gas groups so I can find it easier to find similarities and differences
HapusDo you think why helium boiling point and melting point below room temperature?
BalasHapusThe further down, the noble gas has a denser price, boiling point and an increasing melting point. This is in accordance with the concept of bonding, as the Van Der Walls pull force between the particles will increase.
HapusWhat is the basic form to contrast the element?
BalasHapusWhich distinguishes it from the atomic number, mass number, shape and so does the color
HapusHow to easily distinguish argon and helium by looking at it?
BalasHapusThe difference with looking at the periodic table where swimming difference is the atomic number, its mass number
HapusWhat is the use of helium in everyday life?
BalasHapus1.Helium is used as a filler gas in airships and air balloons, because the gas is lighter than air.
Hapus2. Helium is also used to keep hydrogen-oxygen as a rocket fuel keep in the form of liquid.
3. In addition, helium is used in diving equipment that is as mixed with oxygen because its solubility is very small inside
What is the effect of temperature on a balloon containing helium gas
BalasHapusWhile the buoyancy law does not apply to air (gas), the buoyancy force of air is much smaller than water (liquid) as its relatively low density. In the case of helium, its density is less than the density of atmospheric air. Specifically speaking, the air density is 1.25 g / L, whereas the helium density is only 0.1785 g / L. The high density of atmospheric air can be attributed to the fact that nitrogen (which has a density of 1,2506 g / L) Percent only. Similarly, the weight of the air molecule is 28.9, which is seven times more than that of helium with a molecular weight of 4.0. Like helium, which is used to fill the helium gas balloons, lighter than the outside air, these balloons are subject to buoyancy and start Ascend gradually. Because of the ability of unmanned helium balloons to reach high altitudes, they are often used in atmospheric research to study weather conditions prevailing in the upper atmosphere.
HapusMention the usefulness of argon in daily life ?
BalasHapusBenefits of Argon for Light Bulbs
HapusPerhaps you are wondering if it is true that argon gas is useful in lighting our homes? And the answer is, absolutely right. Argon gas is already widely used in the commercial and industrial world. Light bulbs are just one example of the form of utilization of argon gas for everyday life. Here, argon is used to protect the glow of the bulb because of the natural nature of the Argon gas which is not reactive, allowing argon to retain heat from the light.
Argon is used as a protector in welding
During welding, we will usually see sparks emerging from electrical power. Argon plays an important role in this process because it is used to protect us from sparks produced from the welding process. The working principle is the same as the use of argon on a light bulb, which takes advantage of the basic nature of argon that does not react to heat.
Argon is used in the manufacture of Titanium
Titanium preparations that tend to be reactive, also utilize the role of argon as a blanket or protector. So argon is useful to avoid the titanium from rubbing against the metal in the process of dusting. This is because argon is a protective or inert gas so it can keep the titanium reactive.
Argon is useful in medicine
In addition to the world of welding, argon also can be utilized in the medical world. In fact, the benefits of argon have been felt by people who are in the world of medicine. No wonder indeed, argon is very useful as a protective gas medical devices. In terms of treating illness, argon has its own role that is used in treating patients with deadly diseases, cancer.
Killing Cancer Cells
In China, argon gas has been used in the treatment of cancer.
Argon is used as a medium to kill cancer cells. The trick is to inject this argon gas directly on cancer cells with very low temperatures that are below zero degrees Celsius so that the cancer cells are frozen and will slowly die.
In a special hospital of cancer patients in China has been using argon in the treatment of cancer since 2000. And the safety of the use of argon has been tested and recognized in the world of international medicine.